Bioequivalence of Combination Products: Special Testing Challenges
 Jan, 20 2026
Jan, 20 2026
When a patient takes a pill that contains two medicines in one tablet-like a blood pressure drug paired with a diuretic-it’s called a fixed-dose combination (FDC). These aren’t just convenient. They’re critical for millions managing chronic conditions like HIV, diabetes, or asthma. But getting a generic version of these pills approved? That’s where things get messy. The science behind proving two combination products work the same way in the body-called bioequivalence-is far more complicated than for single-drug pills. And right now, the system is struggling to keep up.
Why Bioequivalence Matters for Combination Products
Bioequivalence isn’t about whether a drug works. It’s about whether it works the same way, at the same speed, and in the same amount. For a single-ingredient tablet, regulators like the FDA or EMA can measure how much of the drug enters the bloodstream and how fast. Simple. For a combination product? You’re tracking two, sometimes three, active ingredients that might interact with each other. One might slow down the absorption of the other. One might break down faster in the gut. That changes everything.
The goal? Let generic manufacturers produce cheaper versions without running full clinical trials on thousands of patients. That’s how we save billions-$373 billion in the U.S. in 2020 alone. But if the generic doesn’t deliver the right amount of each drug at the right time, patients could get too little-or too much-of one component. That’s dangerous.
Fixed-Dose Combinations: The Hidden Interactions
Imagine two drugs that work fine on their own. Put them together in a tablet, and suddenly, one changes how the other dissolves. This isn’t theoretical. It’s happened. In 2022, a generic version of a popular FDC for hypertension failed its bioequivalence test because the diuretic component coated the other drug, preventing full absorption. The company had to reformulate the entire tablet.
Regulators now require generic makers to prove bioequivalence not just to the branded combination product, but also to each individual drug given separately. That means running three-way crossover studies-where volunteers take the brand product, the generic, and each single drug in random order. These studies need 40 to 60 healthy volunteers, not the usual 24 for a single-drug test. And the statistical analysis? It’s more complex. The 80-125% acceptance range for blood concentration (AUC and Cmax) still applies, but only if both drugs hit it. If one falls outside, the whole thing fails.
Failure rates for FDC generics are 25-30% higher than for single drugs, according to experts at the Spanish Medicines Agency. Teva Pharmaceuticals reported that 42% of their complex product development failures were due to bioequivalence issues. And it’s not just about the math. It’s about how the ingredients physically behave in the tablet-how they mix, how they dissolve, how they interact with stomach acid.
Topical Products: Measuring What You Can’t See
Now think about a cream or ointment for eczema that combines a steroid and an antifungal. How do you prove the generic delivers the same amount of each drug into the skin? You can’t just swallow it and measure blood levels. The drug has to penetrate the stratum corneum-the top layer of skin-to work. That’s where tape-stripping comes in. Scientists peel off 15-20 thin layers of skin with adhesive tape and measure how much drug is in each layer.
But here’s the problem: there’s no standard on how deep to go or how much tape to use. One lab might stop at layer 10. Another goes to 18. The results? They don’t match. A generic version of calcipotriene/betamethasone foam failed three times in a row because each bioequivalence study showed wildly different drug penetration levels. The manufacturer spent over $8 million and two years trying to get it right.
These studies cost $5-10 million each. Compare that to $1-2 million for a standard oral bioequivalence study. Most small generic companies can’t afford it. That’s why only a handful of topical combination generics exist, even when the brand patent expires.
Drug-Device Combos: It’s Not Just the Drug
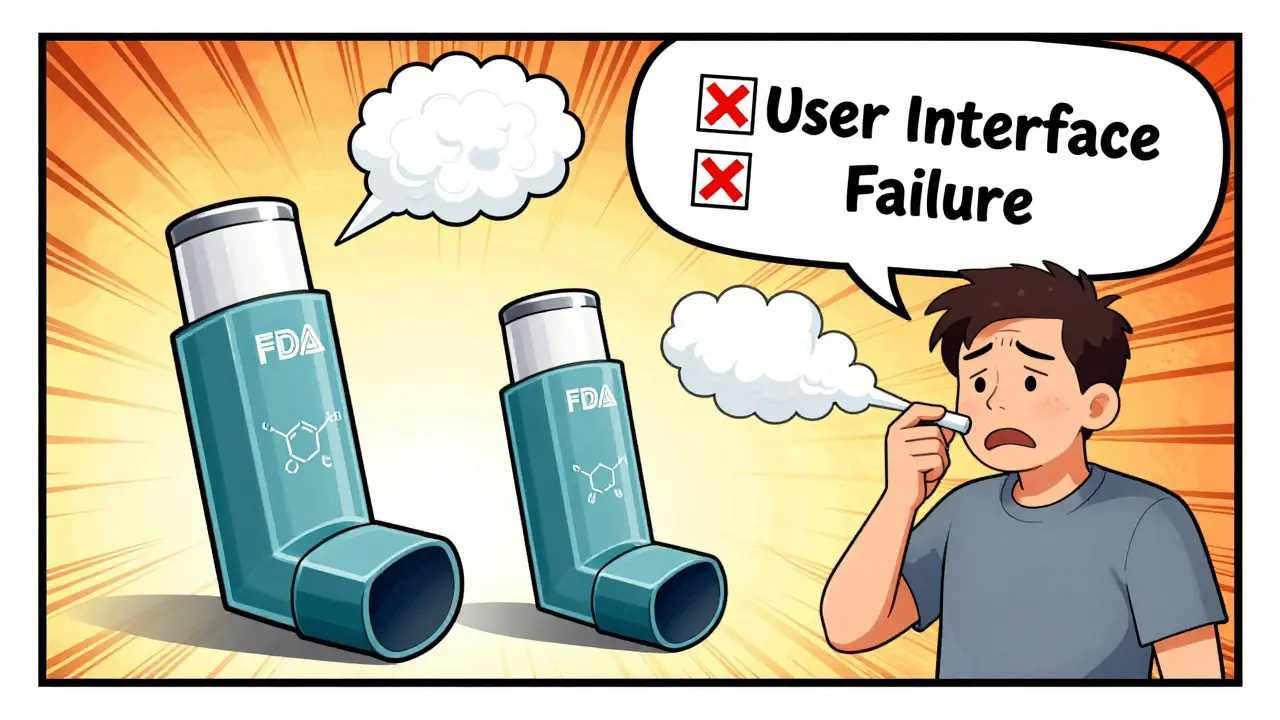
What if the drug is delivered through an inhaler, injector, or nasal spray? Now you’re not just testing the chemical. You’re testing the device. A generic inhaler might have the same active ingredients, but if the button feels stiffer, the aerosol cloud is narrower, or the timing of the puff is off by 0.1 seconds-patients get less medicine. And that’s enough to make it ineffective.
The FDA requires aerosol particle size distribution to be within 80-120% of the brand product. That’s hard to measure. It requires specialized equipment costing over $200,000. And even then, results vary between labs. In 2024, William Doub from the FDA’s Division of Complex Drug Products said 65% of complete response letters for generic inhalers and injectors cite problems with user interface testing. That’s not about chemistry. It’s about how the patient uses it.
One company spent 18 months redesigning the mouthpiece on a generic asthma inhaler just to match the feel of the brand. The active ingredients were identical. The device wasn’t. And that made all the difference.
The Cost and Time Burden
Developing a generic combination product takes 3-5 years and $15-25 million. Bioequivalence testing alone eats up 30-40% of that budget. For small companies, that’s a dealbreaker. The FDA’s Complex Generic Drug Products list includes 312 products as of mid-2024. Only about 40 have approved generics. The rest? Still under patent or too hard to copy.
Approval times are brutal. Standard generics get approved in 14.5 months. Complex products? 38.2 months. And that’s after the company survives multiple rounds of feedback from different FDA divisions. One company submitted the same FDC application to three different review teams and got three different sets of requirements. No wonder 78 industry submissions between 2021 and 2023 called the lack of clear pathways the biggest barrier.
What’s Changing? New Tools and Guidance
There’s hope. The FDA’s Complex Product Consortium has created 12 product-specific bioequivalence guidelines since 2021. Companies using them cut development time by 8-12 months. New tools are helping too.
Physiologically-based pharmacokinetic (PBPK) modeling lets scientists simulate how drugs behave in the body using computer models. It’s not perfect-but it’s accepted in 17 approved generics. One company used PBPK to reduce its clinical study size by 40%, saving $4 million.
For topical products, researchers are linking in vitro tape-stripping data to actual in vivo results. Pilot studies show 85% accuracy in predicting skin delivery from lab measurements. If this scales, it could cut development time and cost dramatically.
The FDA is also working with NIST to create reference standards-like calibrated samples-that labs can use to make sure their measurements match. Initial standards for inhalers are due by late 2024.
What’s Next?
The global market for complex generics hit $112.7 billion in 2023 and is growing fast. But if we don’t fix the bioequivalence system, 45% of these products could stay without generic competition until 2030. That means patients pay more. Hospitals pay more. Insurance companies pay more.
The future isn’t just about better tests. It’s about smarter rules. Product-specific guidance. Standardized methods. Shared reference materials. And a willingness to accept that one-size-fits-all bioequivalence doesn’t work anymore.
For patients, the goal is simple: safe, affordable access to the medicines they need. For regulators and manufacturers, the challenge is making that possible without cutting corners on science.
Why can’t generic combination products just copy the brand’s formula?
Because the ingredients interact. Two drugs that work fine alone may change how each other dissolves, absorbs, or breaks down when combined. A generic company can’t just copy the brand’s recipe-it has to prove the final product behaves the same way in the body. That often means reformulating the entire product, not just matching the ingredients.
Are bioequivalence standards the same worldwide?
No. The FDA and EMA have similar goals but different methods. The EMA often requires additional clinical data for combination products, especially topical and inhaled ones. This duplication adds 15-20% to development costs and delays global launches. There’s no global standard yet, which makes it harder for small companies to enter multiple markets.
How do you test bioequivalence for a cream applied to the skin?
The most common method is tape-stripping: applying adhesive tape to the skin, peeling it off, and measuring how much drug is in each layer. But there’s no standard on how many layers to take or how deep to go. That’s why results vary between labs. New research is linking these lab measurements to actual drug delivery in the skin using predictive models, which could make testing more consistent.
Why do inhaler generics fail so often?
Because the device matters as much as the drug. If the button feels different, the spray pattern changes, or the timing of the puff is off, patients get less medicine. Generic inhalers must match the brand’s aerosol particle size within 80-120%. Even small differences in valve design or propellant can cause failure. Sixty-five percent of FDA rejection letters for inhaler generics cite user interface issues.
Can computer models replace human trials for combination products?
Partially. Physiologically-based pharmacokinetic (PBPK) modeling is now accepted in 17 approved generic applications. It can predict how drugs behave in the body and reduce the need for large clinical studies by 30-50%. But regulators still require some human data, especially for new combinations or high-risk drugs. PBPK is a tool to reduce trials, not eliminate them.
What’s the biggest barrier for small generic companies?
Cost and uncertainty. Developing a combination generic costs $15-25 million and takes 3-5 years. Small companies can’t afford multiple failed bioequivalence studies. Plus, regulatory feedback is inconsistent. One FDA division might accept a method another rejects. That unpredictability makes investment risky. Eighty-nine percent of generic manufacturers say current bioequivalence requirements are unreasonably challenging for complex products.
Steve Hesketh
January 21, 2026 AT 16:11This is one of those topics that doesn’t get enough airtime but literally saves lives. I’ve seen patients switch to generics and keep their meds affordable-no more choosing between rent and refills. The science is wild, but the human impact? Pure gold.
shubham rathee
January 23, 2026 AT 01:23they say its about science but really its just big pharma keeping prices high and the fda in their pocket. why do you think the same companies make the brand and the generic? same people same labs same lies. its all rigged.
MAHENDRA MEGHWAL
January 24, 2026 AT 04:07While the technical challenges outlined in this post are undeniably complex, it is imperative that regulatory frameworks evolve in tandem with pharmaceutical innovation. The current system, while rigorous, may inadvertently stifle equitable access to life-saving medications. A more harmonized international approach to bioequivalence standards could significantly reduce duplication of efforts and accelerate patient access.
Sangeeta Isaac
January 25, 2026 AT 21:23so like… the fda is basically asking generic makers to replicate a magic potion they don’t even understand? and if the pill tastes slightly off? fail. if the inhaler button clicks 0.02 seconds later? fail. we’re treating medicine like it’s a luxury watch and not a lifesaver. also why is no one talking about how this costs $25M per product? that’s not science, that’s corporate extortion.
Alex Carletti Gouvea
January 27, 2026 AT 04:32USA makes the best drugs in the world. Why are we letting other countries dictate how we test them? If we want cheap generics, we need to stop overcomplicating things. Just let them copy it. If someone dies, sue the company. That’s capitalism.
Uju Megafu
January 27, 2026 AT 07:33Oh please. This whole bioequivalence mess is just another way for the FDA to flex power. They don’t care about patients-they care about control. Look at how many times they rejected the same product. That’s not science. That’s bureaucratic sadism. And don’t even get me started on the $8 million spent on tape-stripping. Someone’s getting rich off this chaos.
lokesh prasanth
January 27, 2026 AT 15:50physics is the real drug. not the pill. the interaction is the medicine. not the molecule. we’re measuring shadows and calling it truth.
MARILYN ONEILL
January 27, 2026 AT 20:55Wow. I didn’t know medicine was this complicated. I thought pills were just pills. So now we need computers and tape strips and $25 million just to make a cheaper version? I guess I’ll just keep paying $500 for my asthma inhaler. At least I know it’s the real thing.
Coral Bosley
January 28, 2026 AT 20:17I used to work in a pharmacy. Saw a man cry because he couldn’t afford his combo pill. Then he got the generic. Took it. Had a seizure. We didn’t know why. Turns out the generic had 15% less of one drug. He survived. But I didn’t sleep for a week. This isn’t about science. It’s about who gets to live.
Kevin Narvaes
January 30, 2026 AT 12:16we’re all just atoms in a machine. the drugs, the regulators, the patients. none of us are real. the pill is a symbol. the bioequivalence? a myth. we’re chasing ghosts in a lab coat.
Dee Monroe
January 31, 2026 AT 12:25It’s fascinating to consider how deeply our healthcare system is shaped by the tension between innovation and accessibility. On one hand, we have a responsibility to ensure that every medication, no matter how complex, is safe and effective. On the other, we have a moral obligation to make those medications available to everyone who needs them, regardless of income or geography. The path forward isn’t about choosing one over the other-it’s about reimagining the entire framework. Maybe instead of trying to replicate the exact behavior of a branded product, we could focus on therapeutic equivalence-what actually matters to the patient. Could we design a system where the outcome-stable blood pressure, controlled asthma, reduced viral load-is the metric, not the pharmacokinetic curve? That would require trust, collaboration, and a radical shift in thinking. But isn’t that what medicine is supposed to be about?
Ben McKibbin
February 1, 2026 AT 06:21This is one of those issues where the right thing to do is obvious but politically messy. We need standardized reference materials, yes-but we also need regulators to stop treating every new combination like a nuclear bomb. Let’s create tiered pathways: high-risk drugs get full trials, low-risk ones get PBPK + in vitro. Cut the red tape, keep the science. And for god’s sake, harmonize global standards. Why are we still doing this country-by-country? It’s 2025.
Melanie Pearson
February 2, 2026 AT 11:25While the United States maintains the highest regulatory standards for pharmaceutical products, it is imperative that we preserve these rigorous protocols. Compromising bioequivalence thresholds in the name of cost-efficiency may result in unintended public health consequences. The integrity of our regulatory framework must remain uncompromised, even if it slows the introduction of generic alternatives. Patient safety is not a negotiable variable.
Jerry Rodrigues
February 3, 2026 AT 07:36Been in this field 20 years. The tape-stripping thing? Total mess. But the PBPK models? Game changer. Just give the small guys clear guidelines and a reference standard. They’ll figure it out. No drama needed.
Jarrod Flesch
February 5, 2026 AT 00:20Just had a patient ask me why her generic inhaler didn’t work like the brand. I had to tell her it’s not the drug-it’s the button. She laughed. Then cried. Then asked if we could just make a better one. I didn’t have an answer. 😔
But hey, I’m stoked about the NIST reference standards coming in late 2024. Finally, someone’s listening. 🙌